3CPM, the company responsible for helping develop the non-invasive ENDOSURE TEST for endometriosis, has received the midterm award in the National Institutes of Health (NIH) RADx Tech ACT ENDO Challenge to further develop our Tier-1 technology for at-home endometriosis diagnosis.
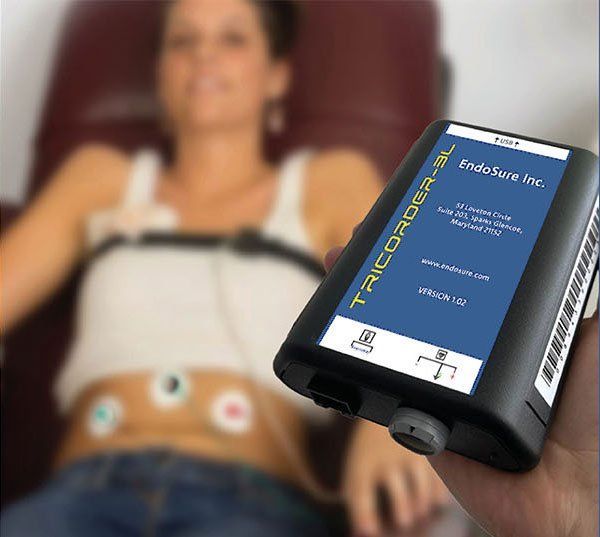
This is a distinctive honor to be one of four finalists in the competition, which ends February 2026. The RADx Tech ACT ENDO Challenge was designed to accelerate the development of innovative, non-invasive diagnostic solutions for endometriosis to facilitate earlier detection of this often-debilitating disease which often presents in non-pelvic areas of the anatomy, such as the brain, thoracic cavity, spine as well as anywhere in the anatomy.
Laparoscopic surgery, largely the standard method of diagnosing endometriosis, is limited because it focuses only on looking inside the abdominal and pelvic cavity. Moreover, it is used primarily to identify only the most advanced stages of the disease, contributing to the delay along with disease progression. ACT ENDO aims to promote new solutions to make earlier diagnosis more accessible, regardless stage of disease in order to reduce the nearly10-year wait women experience before receiving proper diagnosis.
As the only non-invasive, 30-minute, highly accurate test capable of detecting endometriosis at any stage and any location in the body, the ENDOSURE TEST gives healthcare providers earlier insights into the complexities of endometriosis, paving the way for more targeted therapies, post treatment evaluation of therapy, and improved quality of life for millions, while creating a world where no woman has to wait nearly a decade before receiving a diagnosis.
The 3CPM Company has a strategic alliance with ENDOSURE, INC. providing ongoing research and development of the ENDOSURE TEST.
“Kudos, to the NIH for recognizing the need to minimize delay and kudos to Dr. Mark Noar and the 3CPM Company for their achievement to date,” said Mr. Carlos Babini, CEO of ENDOSURE, INC. “We are delighted to report that where the technology is currently used, outcome data, as well as patient and clinician response has been extremely positive. Our mission is to do all we can to help the 1 in 10 women who suffer from this terrible disease with ongoing, compelling research and development.”
Currently, over 200 million women globally are afflicted with endometriosis and bear the many burdens associated with the disease.
Dr. Mark Noar, inventor of the technology and CEO of 3CPM Company states, “Clinicians who offer the ENDOSURE TEST tell me it is a major advancement which enables them to definitively diagnose endometriosis and adenomyosis, further allowing the initiation of treatment at early stages and helping to prevent advancement. The opinion is growing that the technology is in fact, a truly ‘generational’ addition to the FemHealth space. We are appreciative and humbled by this award which will certainly help us move ahead toward future iterations of the TEST. We continue to collaborate with ENDOSURE and do our part on the R&D side to make the many benefits of the ENDOSURE TEST available to more women everywhere.”
Please join us in celebrating this significant achievement and in our efforts to make the ENDOSURE TEST available to women and clinicians around the world.


